Icon row bullet 1:
Pellentesque iaculis sapien eget turpis commodo tempus ipsumelo.
ON-DEMAND
Operational complexity and traditional, siloed systems make eCOA a persistent challenge for study and data management teams.
Ut rutrum, nulla vel hendrerit viverra, nibh ligula feugiat ante, et blandit massa augue id urna. Mauris cursus ipsum in tempus ultrices. Ut nec lacinia ante. Vestibulum ante ipsum primis in faucibus orci luctus et ultrices posuere cubilia curae; Vestibulum nec rutrum quam. Aliquam sollicitudin velit id congue iaculis. In ultricies tristique commodo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do.
Operational complexity and traditional, siloed systems make eCOA a persistent challenge for study and data management teams.
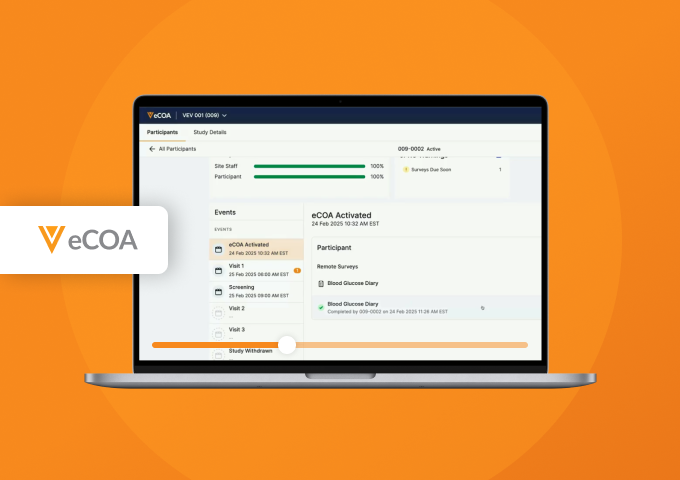
See how Veeva eCOA reduces study build times by 75%, increases mid-study efficiencies, and streamlines data management in this 40-minute product demonstration.
Weekday, Month DD, YYYY |
|
|---|---|
Opening KeynoteLorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. |
00:00 am – 00:00 am PT |
Connect |
00:00 am – 00:00 am PT |
Zone Specific Sessions |
00:00 am – 00:00 am PT |
Weekday, Month DD, YYYY |
|
|---|---|
Closing KeynoteLorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. |
00:00 am – 00:00 am PT |
Aenean lacinia, risus eu lobortis luctus, ante mi tempus ipsum, et vehicula orci neque eu tortor loremedso.
Nam vitae mattis tellus. Donec at purus pellentesque, dignissim nibh et, lobortis lectus. Nullam venenatis lorem auctor.
Nam vitae mattis tellus. Donec at purus pellentesque, dignissim nibh et, lobortis lectus. Nullam venenatis lorem auctor.
Aenean lacinia, risus eu lobortis luctus, ante mi tempus ipsum, et vehicula orci neque eu tortor loremedso.
Aenean lacinia, risus eu lobortis luctus, ante mi tempus ipsum, et vehicula orci neque eu tortor loremedso.
Nam vitae mattis tellus. Donec at purus pellentesque, dignissim nibh et, lobortis lectus. Nullam venenatis lorem auctor.
Pellentesque iaculis sapien eget turpis commodo tempus ipsumelo.
Maecenas placerat venenatis metus a commodo, sed tincidunt sit amet urna lobortis.
Quisque vehicula id elit vel pretium sapien eget turipis.
Pellentesque iaculis sapien eget turpis commodo tempus ipsumelo.
Maecenas placerat venenatis metus a commodo, sed tincidunt sit amet urna lobortis.
Quisque vehicula id elit vel pretium sapien eget turipis.

Title


Title
Company

Title

Title

Title
Company

Title

Title

Title
Company

Title

Title

Senior Director, eCOA Strategy
Veeva Systems
Kieran is responsible for strategies spanning eCOA, eConsent and patient technologies. He's had a long career in clinical trial technology. Prior to Veeva, Kieran spent 14 years at Parexel where he held a variety of positions including establishing Parexel’s RTSM client services team in California, 3 years in Shanghai leading the Parexel Informatics APAC business unit, and as a senior technology leader on sponsor engagements for eClinical and decentralized trials technologies.

Senior Solution Consultant, Clinical Data
Veeva Systems
Alexis plays a pivotal role in enhancing client engagement and optimizing data solutions. She is responsible for identifying customer challenges, demonstrating tailored solutions that meet customer objectives, and delivering business value to the organization. With extensive experience in patient-focused technologies, Alexis has previously held various roles across the clinical technology industry, focusing on product management, roadmap development, and client engagement. Her expertise in the clinical trial industry has been instrumental in helping 30+ companies launch their patient technology initiatives, driving innovation and improving patient outcomes.


















Demo
Sed tincidunt sit amet urna eget placerat. Nulla id sem erat. Quisque vehicu id elit vel pretium. Nam et ex erat. Proin sit amet nibh id nisi porttitor laoreet id ac arcu aenean commodo leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor. Lorem ipsum dolor sit amet, consectetur
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor. Lorem ipsum dolor sit amet, consectetur
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor. Lorem ipsum dolor sit amet, consectetur
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor. Lorem ipsum dolor sit amet, consectetur